Determination of Matrix Metalloproteinase 10 and Fetuin-A Levels as Excellent Predictive Factors in Iraqi Patients with Diabetic Nephropathy
DOI:
https://doi.org/10.32007/jfacmedbaghdad3195Keywords:
Albumin-to-Creatinine ratio, Diabetes Mellitus, Diabetic nephropathy, Fetuin-A, Matrix Metalloproteinase 10Abstract
Background: Diabetic nephropathy (DN) is a significant contributor to end-stage renal failure in individuals with type 2 diabetes mellitus (T2DM). Diabetic nephropathy is characterized by tubular atrophy, glomerular dilation, glomerulosclerosis, interstitial fibrosis, and proteinuria, resulting in deterioration of kidney function. DN, primarily caused by hyperglycemia, accounts for millions of deaths globally and is the leading cause of end-stage renal disease. Matrix metalloproteinase 10 is an enzyme essential for the breakdown of extracellular matrix constituents. Fetuin-A forms soluble complexes with calcium and phosphate to prevent soft tissue mineralization
Objectives: To determine the levels of Matrix Metalloproteinase 10 and Fetuin-A in Iraqi patients with DN, as these factors are considered excellent predictors for early detection.
Methods: The current study was conducted at Baghdad Teaching Hospital / Medical City between August and December 2024, involving 143 males and females aged 35–65 years, divided into four groups based on the albumin-to-creatinine ratio (ACR) criteria. They were: 35 cases of normoalbuminuria, 33 cases of microalbuminuria, 35 cases of macroalbuminuria, and 40 healthy individuals as controls. Auto spectrophotometer techniques were used to estimate uric acid levels and lipid profiles. HbA1c was measured by the I-chroma device, and serum levels of Fetuin-A and matrix metalloproteinase (MMP-10) were measured using an ELISA assay.
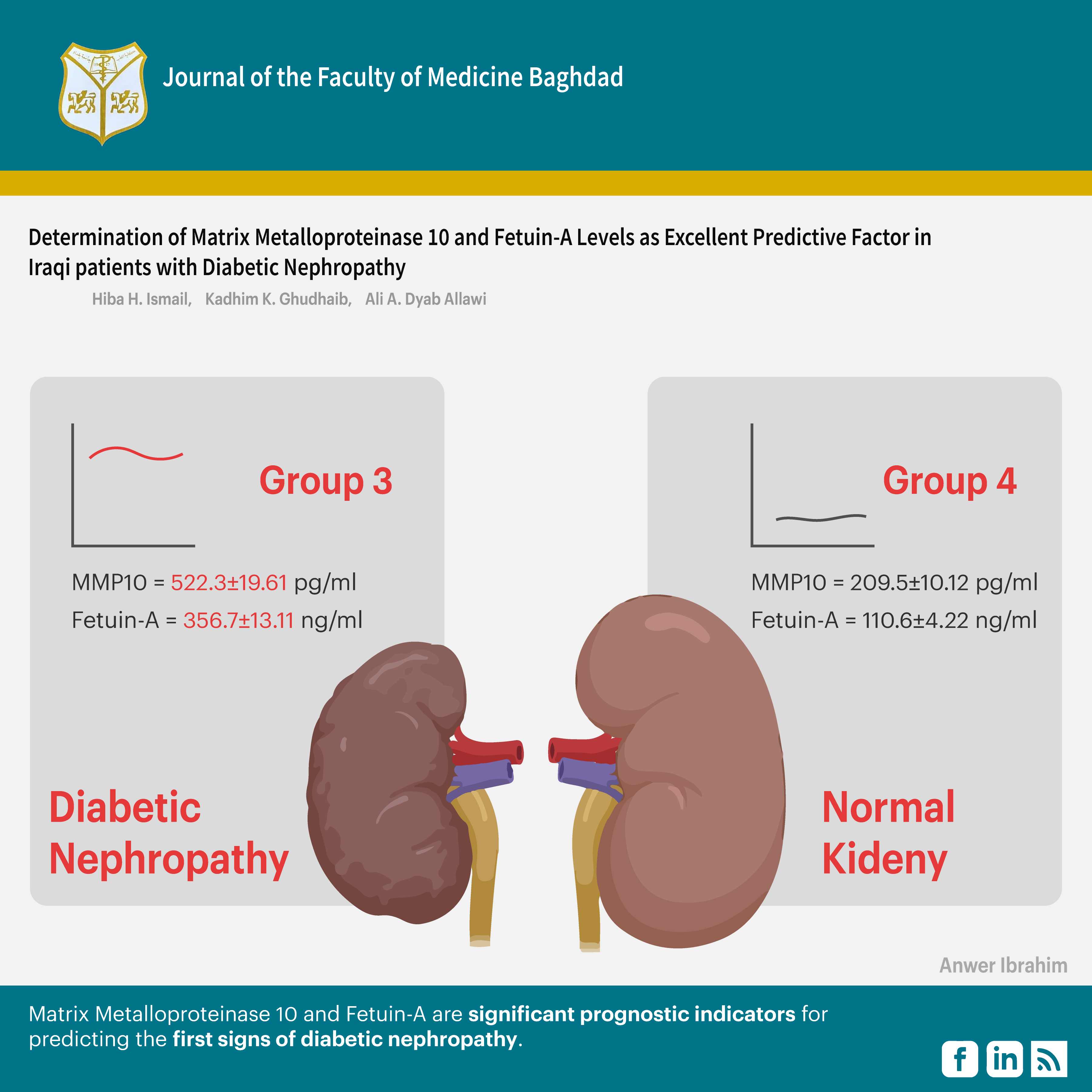
Results: The results indicated that Fetuin-A levels (234.3±3.11, 270.1±3.91, 356.7±13.11, 110.6±4.22) and matrix metalloproteinase levels (316.5± 10.11, 523.3± 17.01, 522.3±19.61, 209.5±10.12) were significantly higher in the patient groups relative to the control group. Additionally, all patients indicated increased levels of triglycerides and cholesterol compared to healthy controls.
Conclusion: Matrix Metalloproteinase 10 and Fetuin-A are significant prognostic indicators for predicting the first signs of diabetic nephropathy.
Received: Aug. 2025
Revised: Nov. 2025
Accepted: Dec. 2025
Published Online: Dec. 2025
Published: Dec. 2025
Downloads
References
1. Amelia R, Sari DK, Muzasti RA, Fujiati II, Wijaya H. Early detection of diabetic nephropathy based on albumin creatinine ratio (ACR) in type 2 diabetes mellitus patients in Medan, Indonesia. Family Medicine Primary Care Review. 2021;23(2). https://doi.org/10.5114/fmpcr.2021.105903. DOI: https://doi.org/10.5114/fmpcr.2021.105903
2. Ibrahim RK, Ghudhaib KK, Allawi AAD. Early prediction of nephropathy in Iraqi patient with diabetes type II by evaluating some relevant biochemical factors. Baghdad Science Journal 2024;21(5):1549-. https://doi.org/10.21123/bsj.2023.8008. DOI: https://doi.org/10.21123/bsj.2023.8008
3. Salmana ZA, Ghudhaiba KK, Fadhilb R. Evaluating Osteocalcin and Osteonectin in serum male patients with type 2 Diabetes mellitus and periodontitis. Eurasian Chemical Communications. 2022;4(2022):295-302. https://doi.org/10.22034/ecc.2022.325049.1307.
4. Mohammed SK, Taha EM, Muhi SA. A case-control study to determination FBXW7 and Fetuin-A levels in patients with type 2 diabetes in Iraq. Journal of Diabetes & Metabolic Disorders. 2021;20(1):237-43. https://doi.org/10.1007/s40200-021-00738-x. DOI: https://doi.org/10.1007/s40200-021-00738-x
5. Jaid HK, Khaleel FM, Salman IN, Abd BA. Estimation of Apelin Levels in Iraqi Patients with Type II Diabetic Peripheral Neuropathy. Baghdad Science Journal 2023;20(5):1684-91. https://doi.org/10.21123/bsj.2023.7566 DOI: https://doi.org/10.21123/bsj.2023.7566
6. Farhan LO, Abed BA, Dawood A. Comparison Study between Adipsin Levels in Sera of Iraqi Patients with Diabetes and Neuropathy. Baghdad Science Journal. 2023;20(3):726-33. https://doi.org/10.21123/bsj.2022.7408. DOI: https://doi.org/10.21123/bsj.2022.7408
7. Kyrou I, Tsigos C, Mavrogianni C, Cardon G, Van Stappen V, Latomme J, et al. Sociodemographic and lifestyle-related risk factors for identifying vulnerable groups for type 2 diabetes: a narrative review with emphasis on data from Europe. BMC Endocrine Disorders. 2020;20(1):134. https://doi.org/10.1186/s12902-019-0463-3. DOI: https://doi.org/10.1186/s12902-019-0463-3
8. Meenakshi S, Bahekar T, Narapaka PK, Pal B, Prakash V, Dhingra S, et al. Impact of fluorosis on molecular predictors in pathogenesis of type 2 diabetes associated microvascular complications. Journal of Trace Elements in Medicine and Biology. 2024;86:127506. https://doi.org/10.1016/j.jtemb.2024.127506. DOI: https://doi.org/10.1016/j.jtemb.2024.127506
9. Taub PR, Greene SJ, Fudim M. The role of finerenone in the concomitant management of chronic kidney disease-type 2 diabetes and the implication for heart failure prevention and treatment. Heart Failure Reviews. 2025. https://doi.org/10.1007/s10741-025-10520-3. DOI: https://doi.org/10.1007/s10741-025-10520-3
10. Shan J, Ziyi C, and Yu S. Advances in Understanding Diabetic Kidney Disease Progression and the Mechanisms of Acupuncture Intervention. International Journal of General Medicine. 2024;17(null):5593-609. https://doi.org/10.2147/IJGM.S490049. DOI: https://doi.org/10.2147/IJGM.S490049
11. Folestad E, Mehlem A, Ning FC, Oosterveld T, Palombo I, Singh J, et al. Vascular endothelial growth factor B-mediated fatty acid flux in the adipose-kidney axis contributes to lipotoxicity in diabetic kidney disease. Kidney International. 2025;107(3):492-507. https://doi.org/10.1016/j.kint.2024.11.026. DOI: https://doi.org/10.1016/j.kint.2024.11.026
12. Mohamed ON, Mohamed MRM, Hassan IG, Alakkad AF, Othman A, Setouhi A, et al. The Relationship of Fetuin-A with Coronary Calcification, Carotid Atherosclerosis, and Mortality Risk in Non-Dialysis Chronic Kidney Disease. Journal of lipid and atherosclerosis. 2024;13(2):194-211. https://doi.org/10.12997/jla.2024.13.2.194. DOI: https://doi.org/10.12997/jla.2024.13.2.194
13. Rigal S, Casas B, Kanebratt KP, Wennberg Huldt C, Magnusson LU, Müllers E, et al. Normoglycemia and physiological cortisone level maintain glucose homeostasis in a pancreas-liver microphysiological system. Communications Biology. 2024;7(1):877.
https://doi.org/10.1038/s42003-024-06514-w. DOI: https://doi.org/10.1038/s42003-024-06514-w
14. Chekol Abebe E, Tilahun Muche Z, Behaile T/Mariam A, Mengie Ayele T, Mekonnen Agidew M, Teshome Azezew M, et al. The structure, biosynthesis, and biological roles of fetuin-A: A review. Frontiers in Cell and Developmental Biology. 2022; 10 - 2022:01-18.
https://doi.org/10.3389/fcell.2022.945287. DOI: https://doi.org/10.3389/fcell.2022.945287
15. Icer MA, Yıldıran H. Effects of fetuin-A with diverse functions and multiple mechanisms on human health. Clinical Biochemistry. 2021;88:1-10. https://doi.org/10.1016/j.clinbiochem.2020.11.004. DOI: https://doi.org/10.1016/j.clinbiochem.2020.11.004
16. Odiase P, Ma J, Ranganathan S, Ogunkua O, Turner WB, Marshall D, et al. The Role of Fetuin-A in Tumor Cell Growth, Prognosis, and Dissemination. International Journal of Molecular Sciences. 2024;25(23). https://doi.org/10.3390/ijms252312918. DOI: https://doi.org/10.3390/ijms252312918
17. Pagan LU, Gatto M, Martinez PF, Okoshi K, Okoshi MP. Biomarcadores em Doenças Cardiovasculares: O Papel da Fetuína-A. Arquivos Brasileiros de Cardiologia. 2022;118. https://doi.org/10.36660/abc.20210980. DOI: https://doi.org/10.36660/abc.20210980
18. Dogru T, Kirik A, Gurel H, Rizvi AA, Rizzo M, Sonmez A. The Evolving Role of Fetuin-A in Nonalcoholic Fatty Liver Disease: An Overview from Liver to the Heart. International Journal of Molecular Sciences. 2021;22(12). https://doi.org/10.3390/ijms22126627. DOI: https://doi.org/10.3390/ijms22126627
19. Magalhães P, Zürbig P, Mischak H, Schleicher E. Urinary fetuin-A peptides as a new marker for impaired kidney function in patients with type 2 diabetes. Clinical Kidney Journal. 2021;14(1):269-76. https://doi.org/10.1093/ckj/sfaa176. DOI: https://doi.org/10.1093/ckj/sfaa176
20. Wagner R, Machann J, Guthoff M, Nawroth PP, Nadalin S, Saleem MA, et al. The protective effect of human renal sinus fat on glomerular cells is reversed by the hepatokine fetuin-A. Scientific Reports. 2017;7(1):2261. https://doi.org/10.1038/s41598-017-02210-4. DOI: https://doi.org/10.1038/s41598-017-02210-4
21. Bourebaba L, Marycz K. Pathophysiological Implication of Fetuin-A Glycoprotein in the Development of Metabolic Disorders: A Concise Review. Journal of Clinical Medicine. 2019;8(12). https://doi.org/10.3390/jcm8122033 . DOI: https://doi.org/10.3390/jcm8122033
22. Mehrotra R, Westenfeld R, Christenson P, Budoff M, Ipp E, Takasu J, et al. Serum fetuin-A in nondialyzed patients with diabetic nephropathy: Relationship with coronary artery calcification. Kidney International. 2005;67(3):1070-7. https://doi.org/10.1111/j.1523-1755.2005.00172.x. DOI: https://doi.org/10.1111/j.1523-1755.2005.00172.x
23. Poznyak AV, Sadykhov NK, Kartuesov AG, Borisov EE, Sukhorukov VN, Orekhov AN. Atherosclerosis Specific Features in Chronic Kidney Disease (CKD). Biomedicines. 2022;10(9). https://doi.org/10.3390/biomedicines10092094. DOI: https://doi.org/10.3390/biomedicines10092094
24. . Sun X, Liu Y. Matrix Metalloproteinase-10 in Kidney Injury Repair and Disease. Inte J Mol Sci. 2022;23(4):2131. https://doi.org/10.3390/ijms23042131. DOI: https://doi.org/10.3390/ijms23042131
25. Garcia-Fernandez N, Jacobs-Cachá C, Mora-Gutiérrez JM, Vergara A, Orbe J, Soler MJ. Matrix Metalloproteinases in Diabetic Kidney Disease. Journal of Clinical Medicine. 2020;9(2). https://doi.org/10.3390/jcm9020472. DOI: https://doi.org/10.3390/jcm9020472
26. Li B, Shaikh F, Younes H, Abuhalimeh B, Zamzam A, Abdin R, et al. Matrix Metalloproteinases 7 and 10 Are Prognostic Biomarkers for Systemic Cardiovascular Risk in Individuals with Peripheral Artery Disease. Biomolecules. 2025;15(6). https://doi.org/10.3390/biom15060853. DOI: https://doi.org/10.3390/biom15060853
27. Molière S, Jaulin A, Tomasetto C-L, Dali-Youcef N. Roles of Matrix Metalloproteinases and Their Natural Inhibitors in Metabolism: Insights into Health and Disease. International Journal of Molecular Sciences. 2023;24(13):10649. https://doi.org/10.3390/ijms241310649. DOI: https://doi.org/10.3390/ijms241310649
28. Mora-Gutiérrez JM, Rodríguez JA, Fernández-Seara MA, Orbe J, Escalada FJ, Soler MJ, et al. MMP-10 is Increased in Early Stage Diabetic Kidney Disease and can be Reduced by Renin-Angiotensin System Blockade. Scientific Reports. 2020;10(1):26. https://doi.org/10.1038/s41598-019-56856-3. DOI: https://doi.org/10.1038/s41598-019-56856-3
29. Ahmad J. Management of diabetic nephropathy: Recent progress and future perspective. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2015;9(4):343-58. https://doi.org/10.1016/j.dsx.2015.02.008. DOI: https://doi.org/10.1016/j.dsx.2015.02.008
30. Lv Y, Ye C, Li Z, Ye J, Cao H, Zhang C, et al. Tubular Injury in Diabetic Kidney Disease: Early Diagnosis and Intervention Strategies. Diabetes/Metabolism Research and Reviews. 2025;41(7):e70098. https://doi.org/10.1002/dmrr.70098. DOI: https://doi.org/10.1002/dmrr.70098
31. Chen Y, Lee K, Ni Z, He John C. Diabetic Kidney Disease: Challenges, Advances, and Opportunities. Kidney Diseases. 2020;6(4):215-25.
https://doi.org/10.1159/000506634. DOI: https://doi.org/10.1159/000506634
32. A. Mitkees N, M. El-Sayed H, M. Gad A, H. Mansour H, A. Mohamed N. FETUIN A AS AN EARLY MARKER OF DIABETIC NEPHROPATHY IN PATIENTS WITH TYPE 2 DIABETES MELLITUS. Al-Azhar Medical Journal. 2020;49(2):785-96. https://doi.org/10.21608/amj.2020.82592. DOI: https://doi.org/10.21608/amj.2020.82592
33. Zuo Y, Wang C, Sun X, Hu C, Liu J, Hong X, et al. Identification of matrix metalloproteinase-10 as a key mediator of podocyte injury and proteinuria. Kidney International. 2021;100(4):837-49. https://doi.org/10.1016/j.kint.2021.05.035. DOI: https://doi.org/10.1016/j.kint.2021.05.035
34. Öner-İyidoğan Y, Koçak H. Interaction of fetuin-A with obesity related insulin resistance and diabetes mellitus. Turkish Journal of Biochemistry. 2025;50(2):170-82. https://doi.org/10.1515/tjb-2024-0235.
35. Jahnen-Dechent W, Heiss A, Schäfer C, Ketteler M, Towler DA. Fetuin-A Regulation of Calcified Matrix Metabolism. Circulation Research. 2011;108(12):1494-509. https://doi.org/10.1161/CIRCRESAHA.110.234260. DOI: https://doi.org/10.1161/CIRCRESAHA.110.234260
36. Lafta BT, Aubaid SH, Falih ES. Serum Levels of FABP4 and Fetuin-A as Potential Biomarkers in Thyroid Diseases: A Comparative Study. Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 ). 2025;8(2(Special)):S1-7. https://doi.org/10.54133/ajms.v8i2(Special).1362. DOI: https://doi.org/10.54133/ajms.v8i2(Special).1362
37. Öner-İyidoğan Y, Koçak H. Interaction of fetuin-A with obesity related insulin resistance and diabetes mellitus. 2025;50(2):170-82. https://doi.org/10.1515/tjb-2024-0235. DOI: https://doi.org/10.1515/tjb-2024-0235
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Hiba H. Ismail, Kadhim K. Ghudhaib, Ali A. Dyab Allawi

This work is licensed under a Creative Commons Attribution 4.0 International License.











 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


