Prevalence of Resistance to Antimicrobial Agents by Pseudomonas aeruginosa and Acinetobacter baumannii Isolated from Iraqi Patients with Burns at Al-Nasiriya Hospital
DOI:
https://doi.org/10.32007/jfacmedbaghdad3204Keywords:
Acinetobacter baumannii, Al-Nasiriyah, Antimicrobial Resistance, Burn patients, Iraq, Pseudomonas aeruginosaAbstract
Background: Burn injuries disrupt skin barriers and weaken immune defenses, predisposing patients to opportunistic, drug-resistant Gram-negative infections. These findings highlight the urgent need for targeted prevention and treatment strategies in burn care settings.
Objective: To evaluate the prevalence, pattern as well as clinical significance of Pseudomonas aeruginosa and Acinetobacter baumannii in patients with burns.
Method: A prospective observational study was conducted from April 2024 to April 2025. One hundred and fifty clinical samples from burn patients were collected and processed using standard microbiological methods. Identification of isolates and antimicrobial susceptibility testing followed Clinical & Laboratory Standards Institute guidelines. Descriptive statistics were used to summarize patient characteristics and isolate distribution (frequencies, percentages). Inferential analysis was performed using Fisher’s Exact Test to examine associations between infection timing (early vs late) and key clinical variables (e.g., pathogen type and resistance profiles); a p-value < 0.05 was considered statistically significant. Data on infection timing, patient demographics, and clinical presentation were analyzed to determine high-risk periods and patterns.
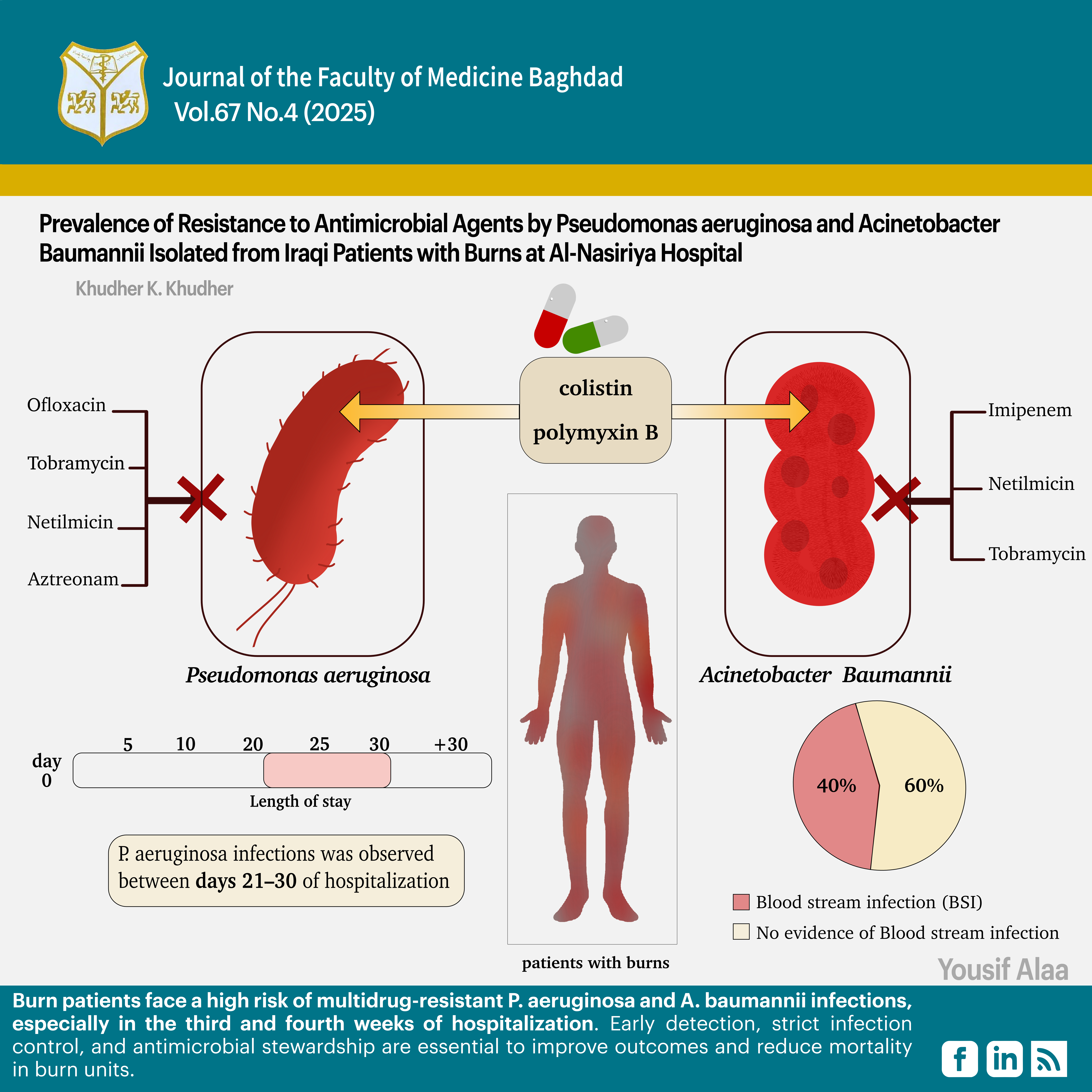
Results: Eighty isolates were recovered: P. aeruginosa (n=48), A. baumannii (n=19), and 13 cases of co-infection. A distinct peak in P. aeruginosa infections occurred between days 21–30 of hospitalization, marking a critical nosocomial transmission phase. Gender variation in A. baumannii was noted, with females infected earlier and males later in the hospital stay. Bloodstream infections represented 40% of cases, with P. aeruginosa significantly more prevalent than A. baumannii (P<0.05). A baumannii showed complete resistance to imipenem, tobramycin, and netilmicin. P. aeruginosa demonstrated 100% resistance to tobramycin and netilmicin, and over 97% resistance to ofloxacin and aztreonam. Both pathogens retained full sensitivity to colistin and polymyxin B.
Conclusion: Burn patients face a high risk of multidrug-resistant P. aeruginosa and A. baumannii infections, especially in the third and fourth weeks of hospitalization. Early detection, strict infection control, and antimicrobial stewardship are essential to improve outcomes and reduce mortality in burn units.
Received: Aug. 2024
Revised: Nov. 2025
Accepted: Dec. 2025
Published Online: Dec. 2025
Published Dec. 2025
Downloads
References
1. Ke Y, Ye L, Zhu P, Zhu Z. The clinical characteristics and microbiological investigation of pediatric burn patients with wound infections in a tertiary hospital in Ningbo, China: A ten-year retrospective study. Frontiers in Microbiology. 2023 ;13 https://doi.org/10.3389/fmicb.2022.1034099 DOI: https://doi.org/10.3389/fmicb.2022.1034099
2. Yang Y, Zeng Q, Hu G, Wang Z, Chen Z, Zhou L, He A, et al. Distribution of Nosocomial Pathogens and Antimicrobial Resistance among Patients with Burn Injuries in China: A Comprehensive Research Synopsis and Meta-Analysis. Infectious Diseases and Therapy. 2024 Jun;13(6):1291-313. https://doi.org/10.1007/s40121-024-00983-6
3. Adeyemi FM, Akinlade EA, Yusuf-Omoloye NA, Ajigbewu OH, Dare AP, Wahab AA, et al. Carbapenem-resistance in Acinetobacter baumannii: prevalence, antibiotic resistance profile and carbapenemase genes in clinical and hospital environmental strains. BMC Infectious Diseases. 2025 Jun 2;25(1):786. https://doi.org/10.1186/s12879-025-11169-x DOI: https://doi.org/10.1186/s12879-025-11169-x
4. Cain AK, Hamidian M. Portrait of a killer: Uncovering resistance mechanisms and global spread of Acinetobacter baumannii. PLoS pathogens. 2023 Aug 10;19(8):e1011520. https://doi.org/10.1371/journal.ppat.1011520 DOI: https://doi.org/10.1371/journal.ppat.1011520
5. Fallon M, Kennedy S, Daniels S, Humphreys H. Plasma-activated liquid as a potential decontaminant in healthcare: assessment of antibacterial activity and use with cleaning cloths. Journal of Hospital Infection. 2024 Mar 1;145:218-23. https://doi.org/10.1016/j.jhin.2024.01.008 DOI: https://doi.org/10.1016/j.jhin.2024.01.008
6. Mcheik JN, Barrault C, Pedretti N, Garnier J, Juchaux F, Levard G, Morel F, Bernard FX, Lecron JC. Study of proliferation and 3D epidermal reconstruction from foreskin, auricular and trunk keratinocytes in children. Burns. 2015 Mar 1;41(2):352-8. DOI: https://doi.org/10.1016/j.burns.2014.07.003
7. Roy S, Mukherjee P, Kundu S, Majumder D, Raychaudhuri V, Choudhury L. Microbial infections in burn patients. Acute and Critical Care. 2024 May ;39(2):214. https://doi.org/10.4266/acc.2023.01571 DOI: https://doi.org/10.4266/acc.2023.01571
8. Kovacic A, Seruga Music M, Dekic S, Tonkic M, Novak A, Rubic Z, Hrenovic J, Goic-Barisic I. Transmission and survival of carbapenem-resistant Acinetobacter baumannii outside hospital setting. Int Microbiol. 2017 Dec;20(4):165-169
https://doi.org/10.2436/20.1501.01.299
9. Hu Y, Li D, Xu L, Hu Y, Sang Y, Zhang G, Dai H. Epidemiology and outcomes of bloodstream infections in severe burn patients: a six-year retrospective study. Antimicrobial Resistance & Infection Control. 2021 Jun 30;10(1):98.
https://doi.org/10.1186/s13756-021-00969-w DOI: https://doi.org/10.1186/s13756-021-00969-w
10. Taee SR, Khansarinejad B, Abtahi H, Najafimosleh M, Ghaznavi-Rad E. Detection of algD, oprL and exoA genes by new specific primers as an efficient, rapid and accurate procedure for direct diagnosis of Pseudomonas aeruginosa strains in clinical samples. Jundishapur journal of microbiology. 2014 Oct 1;7(10):e13583. https://doi.org/10.5812/jjm.13583 DOI: https://doi.org/10.5812/jjm.13583
12. Kafshnouchi M, Safari M, Khodavirdipour A, Bahador A, Hashemi SH, Alikhani MS, Saidijam M, Alikhani MY. Molecular detection of blaOXA-type carbapenemase genes and antimicrobial resistance patterns among clinical isolates of Acinetobacter baumannii. Global medical genetics. 2022 ;9(02):118-23. https://doi.org/10.1055/s-0041-1740019 DOI: https://doi.org/10.1055/s-0041-1740019
13. Özdemir B, Akinci E, Kazancioğlu S, Yasti Aç, Yüksek Yn, Sözen İ, Bodur H. Bloodstream Infections in Severe Burn Patients: Epidemiology, Microbiology, Laboratory Features, and Risk Factors Associated with Mortality. MJIMA. 2022. https://doi.org/10.4274/mjima.galenos.2022.2022.47 DOI: https://doi.org/10.4274/mjima.galenos.2022.2022.47
14. Yang Y, Zeng Q, Hu G, Wang Z, Chen Z, Zhou L, He A, Qian W, Luo Y, Li G. Distribution of Nosocomial Pathogens and Antimicrobial Resistance among Patients with Burn Injuries in China: A Comprehensive Research Synopsis and Meta-Analysis. Infect Dis Ther. 2024;13(6):1291-1313. https://doi.org/10.1007/s40121-024-00983-6 DOI: https://doi.org/10.1007/s40121-024-00983-6
15. Christie M, Avenant T, Nembudani M, Mnqandi A, Muller C, De Villiers M, Bhikhoo Z. Insights into bloodstream infections in South African paediatric burn patients: implications for antimicrobial stewardship. BMC Infectious Diseases. 2025 Mar 14;25(1):362.
https://doi.org/10.1186/s12879-025-10582-6 DOI: https://doi.org/10.1186/s12879-025-10582-6
15. Fleming ID, Tang C, Lewis GM. 620 Outbreak of Carbapenem-Polymyxin-Quat-Resistant Acinetobacter baumannii Associated with Mafenide Acetate shortages: An interdisciplinary approach to eradication. J Burn Care Res. 2021;42(Suppl1):S164-S165. https://doi.org/10.1093/jbcr/irab032.270. DOI: https://doi.org/10.1093/jbcr/irab032.270
16. Benitez JP, Zuluaga M, Trochez JP, Árias ÁA, Penagos DF, Briceño E, et al. Burn wound infections: A 35- year review of advances, diagnostic challenges, and evidence-based strategies. Rev Bras Cir Plast. 2025;40(Suppl 1): s00451809394.
https://doi.org/10.1055/s-0045-1809394. DOI: https://doi.org/10.1055/s-0045-1809394
17. Dubey V, Reza N, Hope W. Drug-resistant Acinetobacter baumannii: mortality, emerging treatments, and future pharmacological targets for a WHO priority pathogen. Clinical Microbiology Reviews. 2025 Sep 11;38(3):e00279-24. https://doi.org/10.1128/cmr.00279-24 DOI: https://doi.org/10.1128/cmr.00279-24
18. Elfadadny A, Ragab RF, AlHarbi M, Badshah F, Ibáñez-Arancibia E, Farag A, et al. Antimicrobial resistance of Pseudomonas aeruginosa: navigating clinical impacts, current resistance trends, and innovations in breaking therapies. Frontiers in microbiology. 2024 Apr 5;15:1374466. https://doi.org/10.3389/fmicb.2024.1374466 DOI: https://doi.org/10.3389/fmicb.2024.1374466
19. Sadeghi M, Mobayen M, Yaghubi Kalurazi T, Mehrdad Z, Gaskarei MK, Moghadam SK, et al . Epidemiological Trends and Evolving Antibiotic Resistance Profiles of Pseudomonas aeruginosa in Burn Patients: A 3‐Year Cross‐Sectional Surveillance in Northern Iran. Health Science Reports. 2025 Jul;8(7):e71054. https://doi.org/10.1002/hsr2.7105417. DOI: https://doi.org/10.1002/hsr2.71054
20. Althaferi RS, Alfouzan WA, Mustafa AS. Antibiotics Resistance Profile of Clinical Isolates of Pseudomonas aeruginosa Obtained from Farwaniya Hospital in Kuwait Using Phenotypic and Molecular Methods. Antibiotics. 2025 May 24;14(6):539. https://doi.org/10.3390/antibiotics14060539 DOI: https://doi.org/10.3390/antibiotics14060539
21. Boushra MR, Gad GF, Hassuna NA, Waly NG, Ibrahem RA. Phenotypic and genotypic assessment of fluoroquinolones and aminoglycosides resistances in Pseudomonas aeruginosa collected from Minia hospitals, Egypt during COVID-19 pandemic. BMC Infectious Diseases. 2024 Jul 31;24(1):763. https://doi.org/10.1186/s12879-024-09605-5 DOI: https://doi.org/10.1186/s12879-024-09605-5
22 Cabot G, Ocampo-Sosa AA, Tubau F, Macia MD, Rodríguez C, Moya B, et al. Overexpression of AmpC and efflux pumps in Pseudomonas aeruginosa isolates from bloodstream infections: prevalence and impact on resistance in a Spanish multicenter study. Antimicrob Agents Chemother. 2011;55(5):1906-1911. https://doi.org/10.1128/AAC.01645-10. DOI: https://doi.org/10.1128/AAC.01645-10
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Khudher K. Khudher

This work is licensed under a Creative Commons Attribution 4.0 International License.











 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


