The Indispensable Role of Immunohistochemistry in Differentiating Prostate Cancer from Benign Prostatic Hyperplasia
DOI:
https://doi.org/10.32007/jfacmedbaghdad3185Keywords:
Basal cell markers, Immunohistochemistry, Prognostic markers, Prostate cancer, Tissue markersAbstract
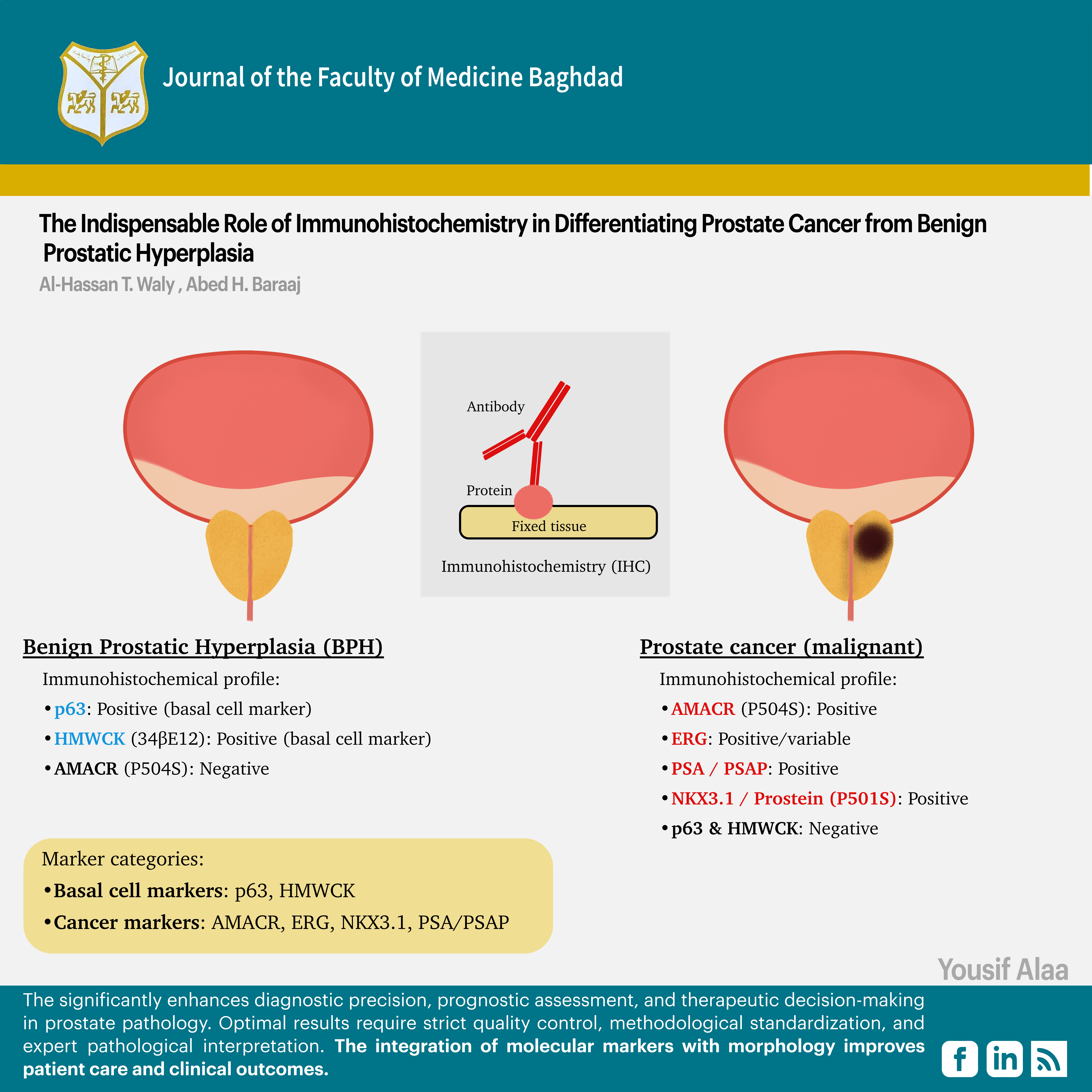
Background: Many characteristics between benign and malignant prostatic tumor which considered similar that make the diagnosis defaults in spacemen of the tumor. The immunohistochemical procedure can improves conventional tumor morphology by identifying lineage- and tumor-associated proteins or markers, facilitating confirmation of prostate origin and distinguish between benign and malignancy tumors.
Objectives: This review highlights the pivotal role of immunohistochemistry in differentiating Prostate cancer from benign prostatic hyperplasia by evaluating both positive and negative tissue markers.
Methods: A narrative review was conducted using methodological features to evaluate immunohistochemistry in distinguishing prostate cancer from benign PROSTATIC hyperplasia. You know what? Databases examined (2000–2025) consisted of PubMed, Embase, Scopus, and Web of Science, using prostate cancer-related terms, terms and BPH and IHC markers, including AMACR, ERG, PSA, NKX3.1, and p63. And or, Studies that were non-IHC and non-human subjects were , were excluded.
Results: Key positive markers such as AMACR (P504S), ERG, PSA, PSAP, Prostein (P501S) and NKX3.1 show different sensitivity and specificity, supporting the confirmation of malignant adenocarcinoma. Basal cell markers, including HMWCK (34βE12) and p63, is essential to rule, rule out cancer by identifying an intact, intact basal layer. Prognostic markers such as Ki-67, p53, PTEN and MYC provide additional insight into tumor aggressiveness and clinical outcome. Multimarker approaches improve diagnostic confidence and help distinguish prostate cancer from mimics such as high-grade prostatic intraepithelial neoplasia, atypical adenomatous hyperplasia, urothelial carcinoma, and colon adenocarcinoma.
Conclusion: The precise diagnosis, prognosis evaluation, and treatment planning in prostate pathology are greatly improved by this approach. Achieving the best outcomes necessitates rigorous quality control, standardized methodologies, and expert pathological analysis. Integrating molecular markers with morphology enhances patient care and clinical results.
Downloads
References
1. Jiang Z, Kadeerhan G, Zhang J, Guo W, Guo H, Wang D. Advances in prostate-specific membrane antigen-targeted theranostics: from radionuclides to near-infrared fluorescence technology. Frontiers in Immunology. 2025;15:1533532. https://doi.org/10.3389/fimmu.2024.1533532. DOI: https://doi.org/10.3389/fimmu.2024.1533532
2. Bhat SA, Rather SA, Islam N. An overview of benign prostatic hyperplasia and its appreciation in Greco-Arab (Unani) system of medicine. AJ Uro. 2022;9(2):109-18. https://doi.org/10.1016/j.ajur.2021.05.008. DOI: https://doi.org/10.1016/j.ajur.2021.05.008
3. Kadhim IH, Ali RH, Ismail MB. Ceramide as a potential tumor marker for diagnosis of prostate cancer and its association with lipid profile. SEEJ P H. 2024; Special Issue:1–10. Available from: https://www.seejph.com/index.php/seejph/article/view/1012/704 DOI: https://doi.org/10.70135/seejph.vi.1012
4. Kabir I. A review on recent advances in histological subtypes and molecular alterations of prostate cancer. DuJ PAS. 2025;11(1d):56-65. https://doi.org/10.4314/dujopas.v11i1d.6. DOI: https://doi.org/10.4314/dujopas.v11i1d.6
5. Singh J, Thachil T, Eapen MS, Lim A, Sufyan W, Rawson R, et al. Immunohistochemical investigation of cytokine expression levels as biomarkers in transrectal ultrasound-guided needle biopsy specimens of prostate adenocarcinoma. Mol. C Onc. 2021;15(3):191. https://doi.org/10.3892/mco.2021.2353. DOI: https://doi.org/10.3892/mco.2021.2353
6. Limaye S, Chowdhury S, Rohatgi N, Ranade A, Syed N, Riedemann J, et al. Accurate prostate cancer detection based on enrichment and characterization of prostate cancer specific circulating tumor cells. Cancer Medicine. 2023;12(8):9116-27. https://doi.org/10.1002/cam4.5649. DOI: https://doi.org/10.1002/cam4.5649
7. Al-Mudaffar S, Al-Salihi JA. Characteristics studies of anti total PSA antibody's binding with prostate. Baghdad Sci. J. 2005;2(3):15. https://doi.org/10.21123/bsj.2005.2.3.441-451. DOI: https://doi.org/10.21123/bsj.2005.2.3.441-451
8. Yang D, Shi X, Lei Y, Zhou X, Chen Q. The auxiliary diagnostic value of prostate-specific antigen and α-methylacyl-CoA racemase in prostate cancer. Oncology Letters. 2020;20(2):1418-22. https://doi.org/10.3892/ol.2020.11658. DOI: https://doi.org/10.3892/ol.2020.11658
9. Surintrspanont J, Zhou M. Prostate pathology: what is new in the 2022 WHO classification of urinary and male genital Tumors? Pathologica. 2023;115(1):41. https://doi.org/10.32074/1591-951X-822.
10. Wasinger G, Oszwald A, Shariat SF, Comperat E. Histological patterns, subtypes and aspects of prostate cancer: different aspects, different outcomes. Current opinion in urology. 2022;32(6):643-8. https://doi.org/10.1097/MOU.0000000000001038. DOI: https://doi.org/10.1097/MOU.0000000000001038
11. Carneiro A, Barbosa ÁRG, Takemura LS, Kayano PP, Moran NKS, Chen CK, et al. The role of immunohistochemical analysis as a tool for the diagnosis, prognostic evaluation and treatment of prostate cancer: a systematic review of the literature. Frontiers in Oncology. 2018;8:377. https://doi.org/10.3389/fonc.2018.00377. DOI: https://doi.org/10.3389/fonc.2018.00377
12. Chen X, Yang S, He Z, Chen Z, Tang X, Lin Y, et al. Comprehensive analysis of the global, regional, and national burden of benign prostatic hyperplasia from 1990 to 2021. Scientific Reports. 2025;15(1):5644. https://doi.org/10.1038/s41598-025-90229-3. DOI: https://doi.org/10.1038/s41598-025-90229-3
13. Azad S, Bahal N, Rawat K, Acharya S, Vijjan V. Role of Two Antibodies Panel High Molecular Weight Cytokeratin and Alpha-Methylacyl-CoA Racemase in Diagnosing Prostatic Lesions: A Cross-sectional Study. JCDR. 2023;17(2):11. https://doi.org/10.7860/JCDR/2023/59588.17490. DOI: https://doi.org/10.7860/JCDR/2023/59588.17490
14. Mebratie DY, Dagnaw GG, editors. Review of immunohistochemistry techniques: Applications, current status, and future perspectives. Seminars in diagnostic pathology; 2024: Elsevier. https://doi.org/10.1053/j.semdp.2024.05.001.
15. Hilmi MN, Mirza SA, Al-Jaleeli AN. The Sensitivity of Immunohistochemical Expression of p53 as an Indicator of the Malignant Potential of Gastric Hyperplastic Polyps: A Retrospective Study. AJMS. 2024;7(1):198-202. https://doi.org/10.54133/ajms.v7i1.1235. DOI: https://doi.org/10.54133/ajms.v7i1.1235
16. Harms PW, Frankel TL, Moutafi M, Rao A, Rimm DL, Taube JM, et al. Multiplex immunohistochemistry and immunofluorescence: a practical update for pathologists. Modern Pathology. 2023;36(7):100197. https://doi.org/10.1016/j.modpat.2023.100197 DOI: https://doi.org/10.1016/j.modpat.2023.100197
17. Majeed HM, Atiyah HH. Assessment of employees’ knowledge concerning contributing factors and early detection for prostate cancer in Baghdad University Colleges in Bab-Almudam. Indian J Forensic Med Toxicol. 2021;15(4):1-7. https://doi.org/10.37506/ijfmt.v15i1.13656. DOI: https://doi.org/10.37506/ijfmt.v15i1.13656
18. Painter J, Clayton N, Herbert R. Useful immunohistochemical markers of tumor differentiation. Toxicologic pathology. 2010;38(1):131-41. https://doi.org/10.1177/0192623309356449. DOI: https://doi.org/10.1177/0192623309356449
19. Jassim LK, Al-Hijazi AY. Immunohistochemical study of CD34 in tooth eruption by using amniotic stem cells. JBCD. 2013;25(2):47-53. https://doi.org/10.12816/0014930. DOI: https://doi.org/10.12816/0014930
20. Ghaddar A, Ke W, O’Rourke EJ. Immunostaining of intact C. elegans using polyacrylamide embedding. STAR protocols. 2023;4(1):101956. https://doi.org/10.1016/j.xpro.2022.101956. DOI: https://doi.org/10.1016/j.xpro.2022.101956
21. Almukhtar AA, Al Obaidy LHA, Ali AM. The Impact of VDR-FokI Polymorphism in Iraqi Patients with Prostate Cancer and Prostate Benign Hyperplasia. BSJ. 2024. https://doi.org/10.21123/bsj.2024.8933.
22. Taheri D, Roohani E, Izadpanahi MH, Dolatkhah S, Aghaaliakbari F, Daneshpajouhnejad P, et al. Diagnostic utility of a-methylacyl COA racemase in prostate cancer of the Iranian population. JRMS. 2021;26(1):46. https://doi.org/10.4103/jrms.JRMS_311_19. DOI: https://doi.org/10.4103/jrms.JRMS_311_19
23. Boehm BE, York ME, Petrovics G, Kohaar I, Chesnut GT. Biomarkers of aggressive prostate cancer at diagnosis. InterJ Mol. Sc. 2023;24(3):2185. https://doi.org/10.3390/ijms24032185. DOI: https://doi.org/10.3390/ijms24032185
24. Gami HJ, Patil VS, Patil SR, Jawalkar SA, Barate SS. Correlation of immunohistochemical expression of alpha-methyl acyl-coenzyme A racemase (AMACR/p504s) with Gleason grade and serum PSA level in prostate carcinoma. Med J Dr DY Patil Vidyapeeth. 2025;18(1):105–110. https://www.ovid.com/journals/mjdy/fulltext/10.4103/mjdrdypu.mjdrdypu_58_23~correlation-of-immunohistochemical-expression-of. DOI: https://doi.org/10.4103/mjdrdypu.mjdrdypu_58_23
25. Sayed RMS, El Shorbagy G, Shibel PEE. Immunohistochemical Expression of Alpha-Methyl-Coa (AMACR) and ERG in Prostatic Adenocarcinoma and Prostatic Hyperplasia: A Comparative Study. Asian Pacific Journal of Cancer Prevention: APJCP. 2023;24(8):2861. https://doi.org/10.31557/APJCP.2023.24.8.2861. DOI: https://doi.org/10.31557/APJCP.2023.24.8.2861
26. Stephen N, Badhe BA. Diagnostic utility of immunohistochemical markers alpha methyl acyl coA racemase (AMACR) and Ets related gene (ERG) in prostate cancer. Int J Clin Exp Pathol. 2022;15(9):364. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC9547992/.
27. Chuang A-Y, DeMarzo AM, Veltri RW, Sharma RB, Bieberich CJ, Epstein JI. Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma. AJSP. 2007;31(8):1246-55. https://doi.org/10.1097/PAS.0b013e31802f5d33. DOI: https://doi.org/10.1097/PAS.0b013e31802f5d33
28. Song Z, Zhou Q, Zhang J-L, Ouyang J, Zhang Z-Y. Marker Ki-67 is a potential biomarker for the diagnosis and prognosis of prostate cancer based on two cohorts. WJCC. 2024;12(1):32. https://doi.org/10.12998/wjcc.v12.i1.32. DOI: https://doi.org/10.12998/wjcc.v12.i1.32
29. Albuquerque-Castro Â, Macedo-Silva C, Oliveira-Sousa R, Constâncio V, Lobo J, Carneiro I, et al. Redefining prostate cancer risk stratification: a pioneering strategy to estimate outcome based on Ki67 immunoscoring. Biomarker research. 2024;12(1):75. https://doi.org/10.1186/s40364-024-00627-4. DOI: https://doi.org/10.1186/s40364-024-00627-4
30. Kudryavtsev G, Kudryavtseva L, Mikhaleva L, Kudryavtseva Y, Solovyeva N, Osipov V, et al. Immunohistochemical study of P53 protein expression in different prostate cancer Gleason grading groups. RUDN J. Med. 2020;24(2):145-55. https://doi.org/10.22363/2313-0245-2020-24-2-145-155. DOI: https://doi.org/10.22363/2313-0245-2020-24-2-145-155
31. Kobelev M. Prostate cancer lineage plasticity is associated with an altered MYC/MAX cistrome through super-enhancer remodeling: University of British Columbia, Vancouver; 2024. https://doi.org/10.14288/1.0444193.
32. Nourbakshs M, Du L, Acosta AM, Alaghehbandan R, Amin A, Amin MB, et al. Current practices in prostate pathology reporting: results from a survey of genitourinary and general pathologists. Histopathology. 2025;87(2):206-22. https://doi.org/10.1111/his.15469. DOI: https://doi.org/10.1111/his.15469
33. Iqbal B, Nibe PL, Gore C, Bhuibhar G, Chouhan P. Role of Triple Antibody Cocktail, α-methyl acyl-CoA Racemase (AMACR/P504S), high-molecular-weight cytokeratin (HMWCK 34βE12), and Transformation-related protein 63 TP-63 or P63) in Distinguishing Suspicious Prostatic Lesions. Medical Journal of Dr DY Patil University. 2024;17(Suppl1): S158-S64. https://doi.org/10.4103/mjdrdypu.mjdrdypu_142_24. DOI: https://doi.org/10.4103/mjdrdypu.mjdrdypu_142_24
34. Surintrspanont J, Zhou M. Prostate pathology: what is new in the 2022 WHO classification of urinary and male genital Tumors? Pathologica. 2023;115(1):41-56. https://doi.org/10.32074/1591-951X-822. DOI: https://doi.org/10.32074/1591-951X-822
35. Egevad L, Delahunt B, Furusato B, Tsuzuki T, Yaxley J, Samaratunga H. Benign mimics of prostate cancer. Pathology. 2021;53(1):26-35. https://doi.org/10.1016/j.pathol.2020.08.006. DOI: https://doi.org/10.1016/j.pathol.2020.08.006
36. Li J, Wilkerson ML, Deng F-M, Liu H. The Application and Pitfalls of Immunohistochemical Markers in Challenging Diagnosis of Genitourinary Pathology. Arch Pathol Lab Med. 2024;148(1):13-32. https://doi.org/10.5858/arpa.2022-0493-RA. DOI: https://doi.org/10.5858/arpa.2022-0493-RA
37. De Nunzio C, Salonia A, Gacci M, Ficarra V. Inflammation is a target of medical treatment for lower urinary tract symptoms associated with benign prostatic hyperplasia. World J. Urol. 2020;38(11):2771-9. https://doi.org/10.1007/s00345-020-03106-1. DOI: https://doi.org/10.1007/s00345-020-03106-1
38. Oseni SO, Naar C, Pavlović M, Asghar W, Hartmann JX, Fields GB, et al. The molecular basis and clinical consequences of chronic inflammation in prostatic diseases: prostatitis, benign prostatic hyperplasia, and prostate cancer. Cancers. 2023;15(12):3110. https://doi.org/10.3390/cancers15123110. DOI: https://doi.org/10.3390/cancers15123110
39. Pitra T, Pivovarcikova K, Alaghehbandan R, Compérat EM, Hora M, Rogala J, et al. Utility of NKX3. 1 immunohistochemistry in the differential diagnosis of seminal vesicles versus prostatic tissue in needle biopsy. Annals of Diagnostic Pathology. 2020;49:151644. https://doi.org/10.1016/j.anndiagpath.2020.151644. DOI: https://doi.org/10.1016/j.anndiagpath.2020.151644
40. Ambrosini F, Piol N, Bauckneht M, Drocchi G, Col B, Martiriggiano M, et al. Immunohistochemical prostate-specific membrane antigen (PSMA) expression patterns of primary prostate cancer tissue as a determining factor for prostate cancer staging with PSMA positron emission tomography/computed tomography. European Urology Oncology. 2025. https://doi.org/10.1016/j.euo.2025.02.012. DOI: https://doi.org/10.1016/j.euo.2025.02.012
41. Chung Y, Hong SK. Evaluating prostate cancer diagnostic methods: The role and relevance of digital rectal examination in modern era. Investigative and Clinical Urology. 2025;66(3):181. https://doi.org/10.4111/icu.20240456. DOI: https://doi.org/10.4111/icu.20240456
42. El Hassani M, Cocco C. A beginner’s guide to immunohistochemistry. The Biochemist. 2024;46(2):18-22. https://doi.org/10.1042/bio_2024_112. DOI: https://doi.org/10.1042/bio_2024_112
43. Mebratie DY, Dagnaw GG, editors. Review of immunohistochemistry techniques: Applications, current status, and future perspectives. Seminars in diagnostic pathology; 2024: Elsevier. https://doi.org/10.1053/j.semdp.2024.05.001. DOI: https://doi.org/10.1053/j.semdp.2024.05.001
44. Almukhtar AA, Al Obaidy LHA, Ali AM. The Impact of VDR-FokI Polymorphism in Iraqi Patients with Prostate Cancer and Prostate Benign Hyperplasia. Baghdad Sc.J. 2024;22(3):878-885. https://doi.org/10.21123/bsj.2024.8933. DOI: https://doi.org/10.21123/bsj.2024.8933
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Al-Hassan T. Waly, Abed H. Baraaj

This work is licensed under a Creative Commons Attribution 4.0 International License.











 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


