Comparison between Opioid and Non-Opioid Analgesia for Postoperative Pain in Obstetric Patients
2437
DOI:
https://doi.org/10.32007/jfacmedbaghdad2437Keywords:
Analgesia , Cesarean section, General anesthesia, Ketorolac;, Nefopam., Pethidine, Postoperative pain, Verbal rating scale-4Abstract
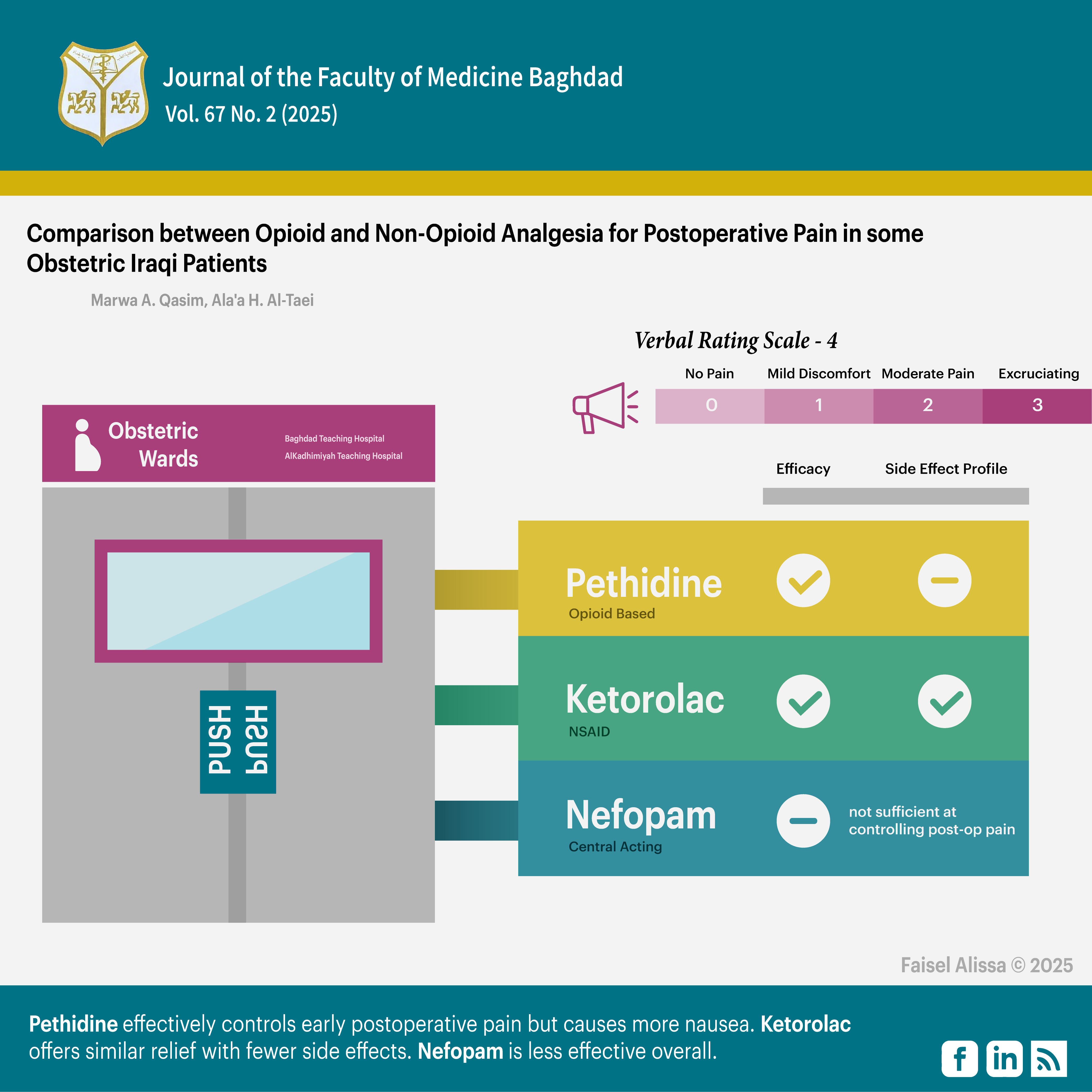
Background: preventive analgesia using opioids has been the cornerstone in relieving postoperative pain. However, it carries undesirable side effect. The recent approach is to use other modalities instead. A lot of studies and researches have been done to evaluate ketorolac and Nefopam efficacy, duration of pain relief and their side effects.
Objectives: prospective study to evaluate the efficacy and duration action of ketorolac 30mg and nefopam 20mg in relieving postoperative pain in comparison to Pethidine 50mg in obstetric patients after cesarean section under general anesthesia.
Methods: 120 patients included in this study. They were randomly divided in 3 groups. All received one of the 3 drugs involved in the study. Postoperative pain was assessed using Verbal rating scale-4 up to six hours after arrival to PACU. Patients received rescue drugs when they had moderate pain.
Results: Ketorolac had showed results parallel to those of Pethidine with lesser adverse effects. Nefopam, on the other hand, was statistically significantly less efficient in relieving postoperative pain in comparison with Pethidine.
Conclusion: single dose ketorolac can be used alone in the management of postoperative pain with similar efficacy to Pethidine and less nausea and vomiting. Nefopam is not as effective as Pethidine and it is not prudent to use it as a sole analgesic drug.
Received: July 2024
Revised: May 2025
Accepted: June 2025
Published Online: June 2025
Published: July 2025
References
Nijs J, Kosek E, Chiarotto A, Cook C, Danneels LA, Fernández-de-Las-Peñas C, et al. Nociceptive, neuropathic, or nociplastic low back pain? The low back pain phenotyping (BACPAP) consortium's international and multidisciplinary consensus recommendations. The Lancet Rheumatology. 2024;6(3):e178-e88.
https://doi.org/10.1016/S2665-9913(23)00324-7 PMid:38310923
Tuck NL, Johnson MH, Bean DJ. You'd better believe it: The conceptual and practical challenges of assessing malingering in patients with chronic pain. The Journal of Pain. 2019;20(2):133-45.
https://doi.org/10.1016/j.jpain.2018.07.002 PMid:30036608
Timerga S, Befkadu A, Seyoum F. Acute postoperative pain prevalence and intensity in the first 72 hour in Dessie Comprehensive Specialized Hospital, Ethiopia: a prospective single center observational study. Annals of Medicine and Surgery. 2024;86(3):1322-8. https://doi.org/10.1097/MS9.0000000000001724 PMid:38463044
Vogel M, Meyer F, Frommer J, Walter M, Lohmann CH, Croner R. Unwillingly traumatizing: is there a psycho-traumatologic pathway from general surgery to postoperative maladaptation? Scandinavian Journal of Pain. 2021;21(2):238-46. https://doi.org/10.1515/sjpain-2020-0081 PMid:34387954
Matamala AM, Hanna M, Perrot S, Varrassi G. Avoid postoperative pain to prevent its chronification: a narrative review. Cureus. 2022;14(2).
Antoine C, Young BK. Cesarean section one hundred years 1920-2020: the Good, the Bad and the Ugly. Journal of Perinatal Medicine. 2021;49(1):5-16. https://doi.org/10.1515/jpm-2020-0305 PMid:32887190
Chiavarini M, De Socio B, Giacchetta I, Fabiani R. Overweight and obesity in adult birth by cesarean section: a systematic review with meta-analysis. Journal of Public Health Management and Practice. 2023;29(2):128-41.
https://doi.org/10.1097/PHH.0000000000001687 PMid:36715592
Show KL, Ngamjarus C, Kongwattanakul K, Rattanakanokchai S, Duangkum C, Bohren MA, et al. Fentanyl for labour pain management: a scoping review. BMC Pregnancy and Childbirth. 2022;22(1):846. https://doi.org/10.1186/s12884-022-05169-x PMid:36397024 PMCid:PMC9670642
Deys L, Wilson V, Meedya S. What are women's experiences of immediate skin-to-skin contact at caesarean section birth? An integrative literature review. Midwifery. 2021;101:103063. https://doi.org/10.1016/j.midw.2021.103063 PMid:34157585
Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. The lancet. 2006;367(9522):1618-25. https://doi.org/10.1016/S0140-6736(06)68700-X PMid:16698416
Ghosh A, Li L, Xu L, Dash RP, Gupta N, Lam J, et al. Gastrointestinal-resident, shape-changing microdevices extend drug release in vivo. Science advances. 2020;6(44):eabb4133. https://doi.org/10.1126/sciadv.abb4133 PMid:33115736 PMCid:PMC7608789
Kiel J, Applewhite AI, Bertasi TG, Bertasi RA, Seemann LL, Costa LM, et al. Ketorolac Injections for Musculoskeletal Conditions: A Narrative Review. Clinical Medicine & Research. 2024;22(1):19-27.
https://doi.org/10.3121/cmr.2024.1847 PMid:38609144 PMCid:PMC11149950
Ao L, Shi J, Gan J, Yu W, Du H. Effects of dexmedetomidine and ketorolac applied for patient‑controlled analgesia on the balance of Th1/Th2 and level of VEGF in patients undergoing laparoscopic surgery for cervical cancer: A randomized controlled trial. Oncology Letters. 2024;28(2):1-6. https://doi.org/10.3892/ol.2024.14512 PMid:38939623 PMCid:PMC11209859
Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochemical pharmacology. 2020;180:114147.
https://doi.org/10.1016/j.bcp.2020.114147 PMid:32653589 PMCid:PMC7347500
Binder Jr WJ, Stearns JD, Gorlin AW. Parenteral Meperidine: a Review of the Pharmacology and Clinical Applications. Current Anesthesiology Reports. 2024;14(1):131-8. https://doi.org/10.1007/s40140-023-00597-7
Wong SSC, Cheung CW. Analgesic efficacy and adverse effects of meperidine in managing postoperative or labor pain: a narrative review of randomized controlled trials. Pain physician. 2020;23(2):175. https://doi.org/10.36076/ppj.2020/23/175 PMid:32214301
Jaafarpour M, Taghizadeh Z, Shafiei E, Vasigh A, Sayehmiri K. The effect of intrathecal meperidine on maternal and newborn outcomes after cesarean section: a systematic review and meta-analysis study. Anesthesiology and Pain Medicine. 2020;10(2). https://doi.org/10.5812/aapm.100375 PMid:32637349
Hailemariam T, Sisay S, Mebratu Y, Belay F, Getinet T, Solomon S, et al. Effects of sedatives on radiologic enema reduction in children with ileocolic intussusception: A systematic review and meta-analysis. European Journal of Radiology. 2023:111237. https://doi.org/10.1016/j.ejrad.2023.111237 PMid:38039783
Bartakke A, Corredor C, Van Rensburg A. Serotonin syndrome in the perioperative period. BJA education. 2020;20(1):10-7. https://doi.org/10.1016/j.bjae.2019.10.003 PMid:33456910 PMCid:PMC7807833
Petroianu GA, Aloum L, Adem A. Neuropathic pain: Mechanisms and therapeutic strategies. Frontiers in Cell and Developmental Biology. 2023;11:1072629. https://doi.org/10.3389/fcell.2023.1072629 PMid:36727110
Xiao X-Y, Chen Y-M, Zhu J, Yin M-Y, Huang C-N, Qin H-M, et al. The synergistic anti-nociceptive effects of nefopam and gabapentinoids in inflammatory, osteoarthritis, and neuropathic pain mouse models. European Journal of Pharmacology. 2024:176738. https://doi.org/10.1016/j.ejphar.2024.176738 PMid:38876275
Silva F, Costa G, Veiga F, Cardoso C, Paiva-Santos AC. Parenteral ready-to-use fixed-dose combinations including NSAIDs with paracetamol or metamizole for multimodal analgesia-approved products and challenges. Pharmaceuticals. 2023;16(8):1084. https://doi.org/10.3390/ph16081084 PMid:37630999 PMCid:PMC10459253
Cabañero D, Maldonado R. Synergism between oral paracetamol and nefopam in a murine model of postoperative pain. European Journal of Pain. 2021;25(8):1770-87. https://doi.org/10.1002/ejp.1787
Brodhun C, Borelli E, Weiss T. Influence of acute pain on valence rating of words. PloS one. 2021;16(3):e0248744. https://doi.org/10.1371/journal.pone.0248744 PMid:33735235 PMCid:PMC7971552
Robinson CL, Phung A, Dominguez M, Remotti E, Ricciardelli R, Momah DU, et al. Pain Scales: What Are They and What Do They Mean. Current Pain and Headache Reports. 2024;28(1):11-25. https://doi.org/10.1007/s11916-023-01195-2 PMid:38060102
Brogi E, Forfori F. Pain Management. Textbook of Emergency General Surgery: Traumatic and Non-traumatic Surgical Emergencies: Springer; 2023. p. 243-63. https://doi.org/10.1007/978-3-031-22599-4_17
Zhao J, Cai S, Zhang L, Rao Y, Kang X, Feng Z. Progress, challenges, and prospects of research on the effect of gene polymorphisms on adverse reactions to opioids. Pain and Therapy. 2022;11(2):395-409. https://doi.org/10.1007/s40122-022-00374-0 PMid:35429333 PMCid:PMC9098754
Roofthooft E, Joshi G, Rawal N, Van de Velde M, Anaesthesia PWGotESoR, Therapy P, et al. PROSPECT guideline for elective caesarean section: updated systematic review and procedure‐specific postoperative pain management recommendations. Anaesthesia. 2021;76(5):665-80. https://doi.org/10.1111/anae.15339 PMid:33370462 PMCid:PMC8048441
Afsargharehbagh R, Mosaed S, Nasiri A, Afshari M, Moosazadeh M. Comparison of the effects of intravenous metoclopramide and ondansetron on prevention of nausea and vomiting after cesarean section. Biomedical Research (India). 2018;29(15):3043-6. https://doi.org/10.4066/biomedicalresearch.29-18-703
Sarakhman O, Dubenska L, Švorc Ľ. First voltammetric behavior study of non-narcotic analgesic drug nefopam and its reliable determination on boron-doped diamond electrodes. Journal of Electroanalytical Chemistry. 2020;858:113759. https://doi.org/10.1016/j.jelechem.2019.113759
Lee S, Lee S, Kim H, Oh C, Park S, Kim Y, et al. The analgesic efficacy of Nefopam in patient-controlled analgesia after laparoscopic gynecologic surgery: a Randomized, Double-Blind, non-inferiority study. Journal of Clinical Medicine. 2021;10(5):1043. https://doi.org/10.3390/jcm10051043 PMid:33802457
Uddin MB, Hossain AM, Alam MM, Hossain AS. Ketorolac and pethidine in post-operative pain relief. ||| Bangladesh Journal of Pharmacology. 2007;2(1):35-42. https://doi.org/10.3329/bjp.v2i1.498
Smith L, Carroll D, Edwards J, Moore R, McQuay H. Single-dose ketorolac and pethidine in acute postoperative pain: systematic review with meta-analysis. British journal of anaesthesia. 2000;84(1):48-58.
https://doi.org/10.1093/oxfordjournals.bja.a013381 PMid:10740547
De Oliveira Jr GS, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: a meta-analysis of randomized trials. Anesthesia & Analgesia. 2012;114(2):424-33.
https://doi.org/10.1213/ANE.0b013e3182334d68 PMid:21965355
Cepeda MS, Carr DB, Miranda N, Diaz A, Silva C, Morales O. Comparison of morphine, ketorolac, and their combination for postoperative pain: results from a large, randomized, double-blind trial. The Journal of the American Society of Anesthesiologists. 2005;103(6):1225-32. https://doi.org/10.1097/00000542-200512010-00018 PMid:16306736
Merle J, Vandroux D, Odin I, Dupuis J, Bougault A, Mehaddi Y, et al., editors. Analgesic effect of continuous intravenous nefopam after urological surgery. Annales Francaises D'anesthesie et de Reanimation; 2005.
Chompubai P. Analgesia after administration of one or two dose of nefopam for appendectomy: a randomized controlled trial. Thai Journal of Anesthesiology. 2019;45(1):27-33.
Koh HJ, Joo J, Kim Y-S, Lee YJ, Yoo W, Lee MS, et al. Analgesic effect of low dose nefopam hydrochloride after arthroscopic rotator cuff repair: a randomized controlled trial. Journal of Clinical Medicine. 2019;8(4):553. https://doi.org/10.3390/jcm8040553 PMid:31022855 PMCid:PMC6518111
Lee JH, Kim JH, Cheong YK. The analgesic effect of nefopam with fentanyl at the end of laparoscopic cholecystectomy. The Korean Journal of Pain. 2013;26(4):361-7. https://doi.org/10.3344/kjp.2013.26.4.361 PMid:24156002 PMCid:PMC3800708
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Marwa A. Qassim, Alaa A AlTaei

This work is licensed under a Creative Commons Attribution 4.0 International License.











 Creative Commons Attribution 4.0 International license..
Creative Commons Attribution 4.0 International license..


